Hannah Totte, MPH and Katelyn Jetelina at Your Local Epidemiologist have assembled this list of public health victories in 2025:
-------------------------------
20 public health wins in 2025: We have your back.
Phew, what a year. Amid relentless political, financial, and rhetorical pressures on public health, science, and health care, real harm landed on clinics, communities, and people trying to stay healthy.
The public health sector did everything it could to mitigate the impact and continue protecting Americans’ health in meaningful, lifesaving ways. Although the blows are becoming increasingly difficult to absorb, outbreaks were still prevented, harm was still reduced, and people were still kept safe.
As we head into the new year, here are 20 wins in 2025 that made our world healthier! Something we can all celebrate.
(Disclaimers: These are in no particular order, and we surely missed many, but thank you to everyone who shared their ideas. Please keep sharing your stories; they show the true power of local public health.)
Triumphs in the field
Over 3,500 local health departments keep your invisible shield intact and strong. Here are a few (of many) wins this year:
Fifty measles outbreaks were contained. This success reflects tireless work by local public health teams and strong community responses, including vaccination. For example, early uptake of the MMR vaccine increased rapidly among Texas infants after the state’s measles outbreak began in January.
Infant botulism outbreak contained. Experts in California, the only group worldwide with access to the antidote, BabyBIG, identified a highly unusual signal in baby formula, triggering rapid notifications to CDC, manufacturers, and suppliers saving infant lives.
Leading on climate adaptation. Maricopa County cut heat-related deaths by nearly 40% in 2025 (the second year of decline on record) even as extreme heat days increased, thanks to expanded cooling centers, hydration stations, and outreach to vulnerable residents.
Turning the tide
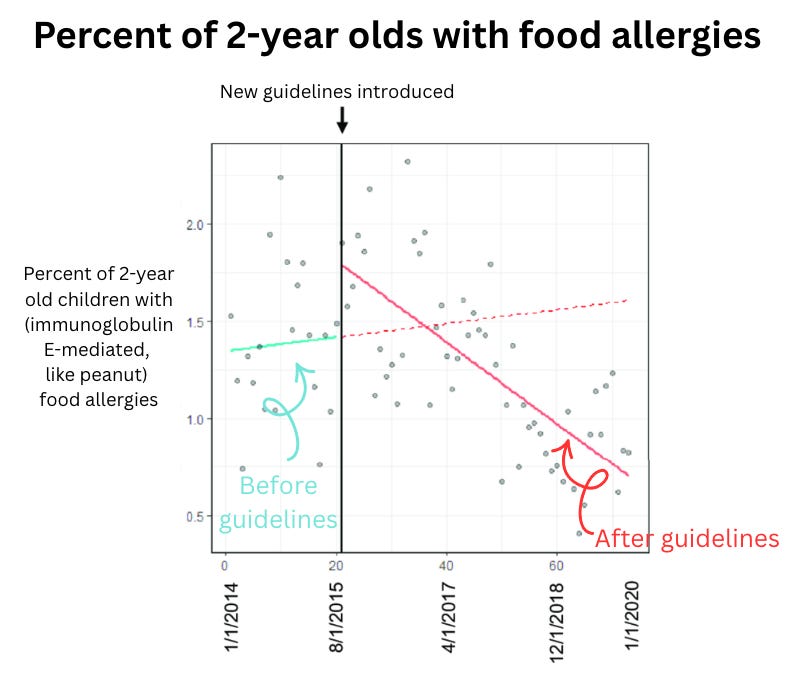
Food allergies in kids dropped dramatically. This year, we got news that childhood food allergies dropped 36%, driven by a 43% drop in peanut allergy. This success traces back to the 2015 LEAP study, which showed that early introduction of potential allergens prevents allergy—changing guidelines and, now, lives. More kids can safely reach for a PB&J.
Huntington’s disease was slowed for the first time. A targeted gene therapy delivered during brain surgery slowed disease progression by ~75%. Disease progression that usually happens in one year took four years instead, which is an extraordinary breakthrough for families facing a devastating disease.
Obesity rates continued to decline. GLP-1 medications likely played a role, but they’re not the whole story. While we don’t yet fully understand the drivers, the direction matters, and it’s good news.
Outreach initiatives improved cancer screening and reduced disparities. A study released this year showed that in a Northern California health system, colorectal cancer screenings doubled over the past 13 years, and deaths due to colorectal cancer dropped by 50% among Black patients.
Policy at play
Menopause hormone therapy (MHT) has become more accessible, with the removal of its black box warning. After 85 years of conflicting research and confusing guidelines, there is hope for women suffering preventable menopause symptoms.
New Mexico guaranteed free child care for all families. No income limits or copays required. A rare, bold move with real public health impact, including economic benefits.
Red flag gun laws expanded. Maine voters passed one, bringing the total to 22 states + D.C. These laws reduce gun deaths—now the work is awareness and implementation.
Maryland made adult vaccines free. A first-of-its-kind program was launched to provide recommended vaccines at no cost for uninsured and underinsured adults. Public health nurses have begun delivering them.
Schools kept kids fed during federal shutdowns. For example, New Hampshire districts expanded free meals amid SNAP disruptions, preventing hunger when families needed support most.
International successes
86 million girls in high-risk countries have received the HPV vaccine. That’s an estimated 1.4 million lives saved. By year’s end, countries that bear 89% of the global burden of cervical cancer will have access to the HPV vaccine.
HIV prevention shots became affordable. Lenacapavir—nearly 100% effective—will cost $40/year in 120 low- and middle-income countries by 2027, down from $42,000.
PEPFAR survived. $400 million in global HIV and AIDS funding was preserved. Since 2003, this bipartisan program has saved 26 million lives and enabled 7.8 million HIV-free births.
The first-ever malaria treatment for newborns was approved, filling a deadly treatment gap. It will be distributed by a nonprofit starting in eight African countries.
Standing up for science
This year, our field showed remarkable resilience more than ever before. Here are a few highlights.
New coalitions formed nationwide, filling gaps, staying rooted in evidence, and working to ensure Americans feel confident and protected.
The Vaccine Integrity Project gives Americans independent, third-party confirmation that vaccines are safe and effective.
Northeast and Western state public health coalitions now coordinate health guidance so residents get clear, consistent recommendations.
GovAct is helping governors protect Americans’ health freedoms through coordinated action.
Grandparents for Vaccines, a grassroots group, is sharing real-life stories of how vaccines have protected children and families.
The Evidence Collective, cofounded by YLE, is uniting researchers, communicators, and practitioners to turn rigorous science into clear, actionable guidance that people can use.
Lawsuits defended evidence-informed processes.
The American Academy of Pediatrics and other medical societies sued HHS over unilateral changes to vaccine policy and the restructuring of CDC advisory processes.
Coalitions of clinicians and public health organizations filed lawsuits to stop the removal of federal public health data and clinical resources.
Researchers and public health groups sued the National Institutes of Health (NIH) and HHS to challenge the cancellation of peer-reviewed research grants.
Epidemiologists entered politics. Scientists entered the arena and are running for office, breaking long-standing silos and bringing evidence into policymaking, where it’s desperately needed.
Courage at CDC, NIH, and beyond. CDC leaders resigned to take a stand against what their leadership was asking them to do. NIH employees wrote a declaration. Federal, state, and local public health workers continue to take silent and public stands every day through their work.
YLE wins—because community matters
This year was intense for YLE, but you helped us grow and build like never before. This year we:
Launched a California newsletter with Dr. Matt Willis.
Hosted our first in-person event (yes, at a comedy club) with YLE New York’s Marisa Donnelly.
Welcomed incredible teammates: Celeste (COO), Nat (Data Innovation Lead), and Hannah (Community Manager).
Built unlikely collaborations rooted in shared values, like with MAHA and mom influencers.
Launched Project Stethoscope and The Evidence Collective.
Published more than 120 newsletters and hosted 4 webinars.
Above all, we built a community with nearly 415,000 subscribers. (Tell a friend and help us reach that milestone this year!)
Bottom line
Public health has your back.
This field will be challenged like never before in 2026, but I’m confident it can navigate this terrain with relentless dedication, innovation, partnership, and listening.
Here’s to 2026. Happy New Year!
Love, YLE


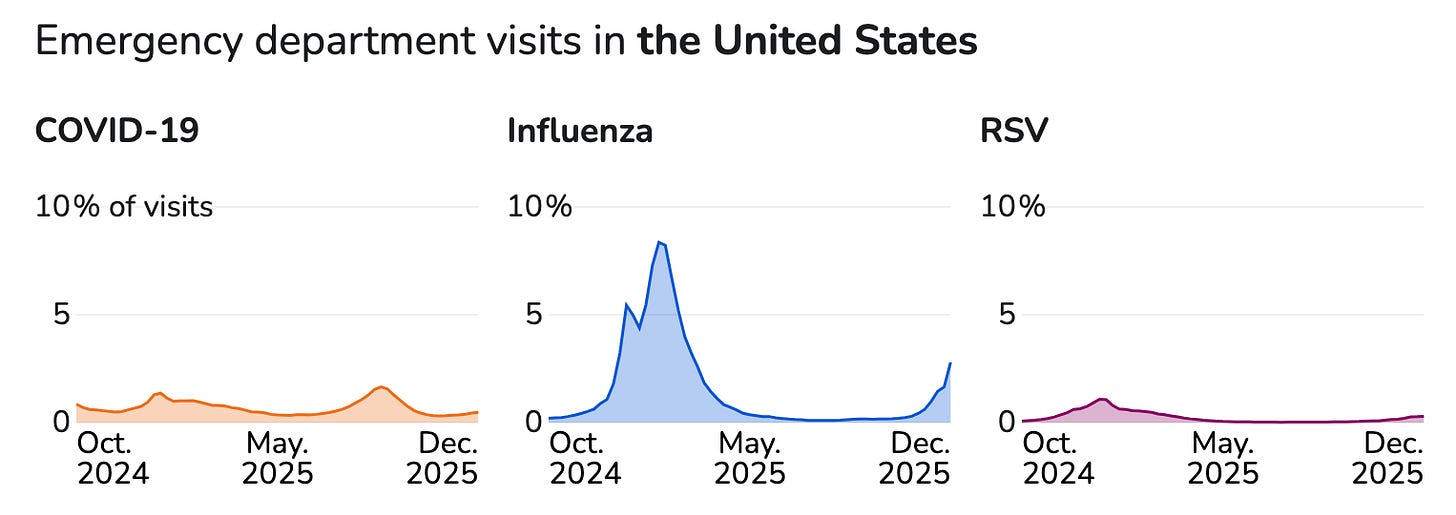
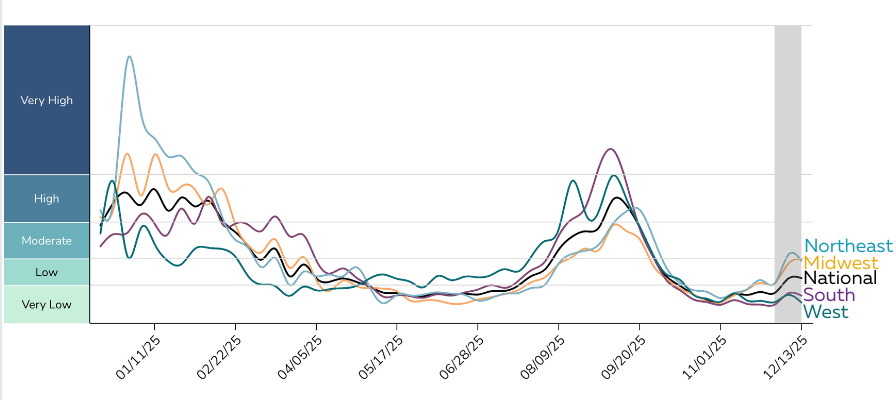














.jpg)