Caitlin Rivers, Aug 19, 2025
"Fall vaccination season is fast approaching, and this year brings both familiar recommendations and some important changes. This guide shares what I was able to uncover about flu, Covid-19 and RSV vaccines for the 2025-2026 season, including eligibility, timing, and new options like at-home flu vaccines. This was a challenging topic to research so while I've done my best to provide accurate information, there may be updates or additional information in the weeks ahead.
Will the flu vaccine be available?
Yes. Both the FDA and CDC processes required to bring the seasonal influenza vaccine to market are complete.
Who should get it and when?
The annual flu vaccine is recommended for everyone over the age of 6 months. Getting it as soon as possible is not necessary better, because activity doesn’t really pick up until later in autumn. The best time to get vaccinated is by the end of October.
Adults age 65+ would benefit from a special flu vaccine that provides better protection. The CDC recommends these stronger vaccines for older adults:
Fluzone High-Dose
Flublok
Fluad
Children 6 months to 8 years need 2 doses (given 4+ weeks apart) if:
They've never had a flu vaccine before, OR
Their vaccination history is unknown, OR
They haven't previously received at least 2 flu vaccines given 4+ weeks apart
What’s new with flu vaccines?
There is a new, at-home option. FluMist, a live-attenuated flu vaccine that is sprayed up the nose rather than given as a shot, can be administered at home by people 2-49. It is not available in all states. According to the AstraZeneca website, here is how you can get the vaccine:
Individuals 18 and older can visit www.FluMist.com to learn more and click to order, where they will be directed to complete a medical screening questionnaire. A licensed healthcare provider will review each submission to determine eligibility. Once eligibility is confirmed and insurance is verified, FLUMIST will be prescribed and shipped directly to the consumer’s home on the date they had selected, complete with clear administration instructions, storage guidance and how to dispose.
Will it the Covid-19 vaccine be approved?
FDA has not yet approved the 2025-2026 vaccine formulation, but it's not too late based on historical timing. We should have more clarity by the end of August. The vaccine approval process follows these steps.
Strain selection. The new formulation to match circulating variants is chosen. This step is complete.
Manufacturing. The vaccine is manufactured by e.g., Pfizer and Moderna. Presumably complete.
FDA approval. FDA must approve the updated vaccine with new labeling. This is usually done in August, and is still pending. We are here.
Historical FDA approval dates show there's still time for FDA approval. Previous approvals came on September 11, 2021, August 31, 2022 and August 22, 2023.
CDC meets to develop recommendations for who should get the vaccine and when. ACIP has a meeting tentatively scheduled for August or September 2025, though no specific date has been announced.
Final CDC sign-off. After ACIP votes, the CDC Director signs off on the recommendations, making them official policy.
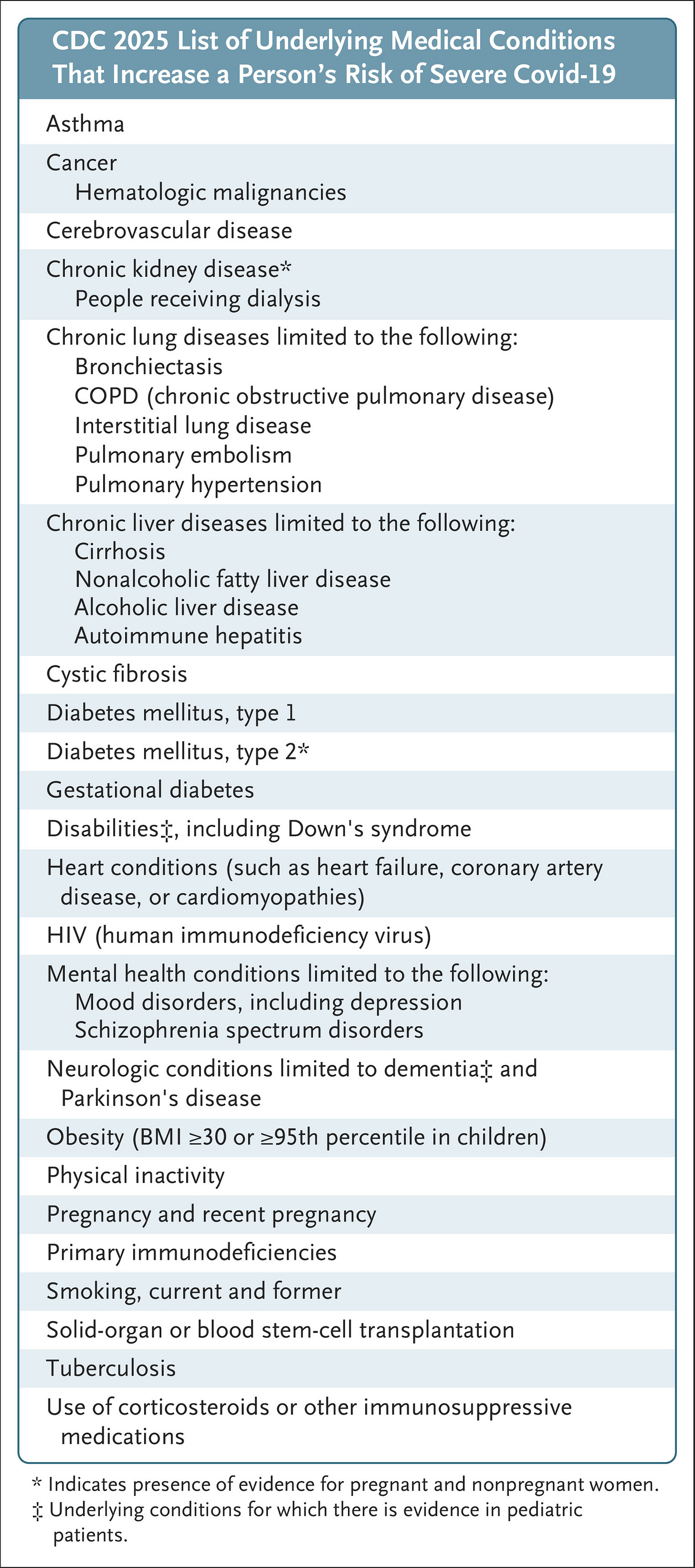
Who will be eligible to receive the vaccine?
Best guess: older adults AND people with health conditions. Approval will almost certainly be limited to adults over the age of 65 years and people of any age with risk factors for severe illness. The qualifying conditions are very broad, so take a close look even if you don’t think of yourself as having a health condition. For example, obesity, depression and diabetes are all on the list.
Children under 5 with no health conditions may not have any options. The CDC has reportedly sent emails to state and local health departments informing them that the FDA is considering ending Pfizer’s authorization for this age group this fall. There are no other Covid-19 vaccines currently approved for healthy children in this age group. Moderna’s vaccine is currently approved for children 6 months and older who have a qualifying health condition.
I want to get vaccinated but I won’t be eligible. Should I get it now?
There are a few things to consider here. The benefits to getting vaccinated now are:
Less uncertainty about access issues and insurance payments.
The summer wave is underway, whereas it will likely be past peak in a month.
The drawbacks:
The currently-available vaccine is last season’s formulation, which may not be as good of a match to the variants currently circulating.
There may be access issues even now. The approval for last season’s Moderna vaccine, for example, has already been limited to the high risk groups. I did not find any evidence that Pfizers is limited, but I can’t be certain.
What about insurance coverage?
Non-priority groups may still have insurance coverage (uncertain!). AHIP, the trade association representing health insurers, issued a press release in June reaffirming their commitment to covering vaccines: “We are committed to ongoing coverage of vaccines to ensure access and affordability for this respiratory virus season. We encourage all Americans to talk to their health care provider about vaccines.”
Also, the Associated Press reported in May that Blue Cross Blue Shield planned to continue covering the vaccine.
Neither of these pieces of information are conclusive, but they do suggest that insurance coverage may continue even if eligibility is narrowed.
Notes for people who are immunocompromised
Don’t forget that there are additional treatment and prevention options for people who are immunocompromised:
Last year, FDA authorized an infusion called Pemgarda for people who “are unlikely to mount an adequate immune response to COVID-19 vaccination.” It’s given at least two weeks after vaccination to augment protection.
People who are immunocompromised may also be able to get additional doses of the vaccine, at least two months after their last dose.
Paxlovid is also still available. It’s a treatment for people who are infected and at high risk of severe illness.
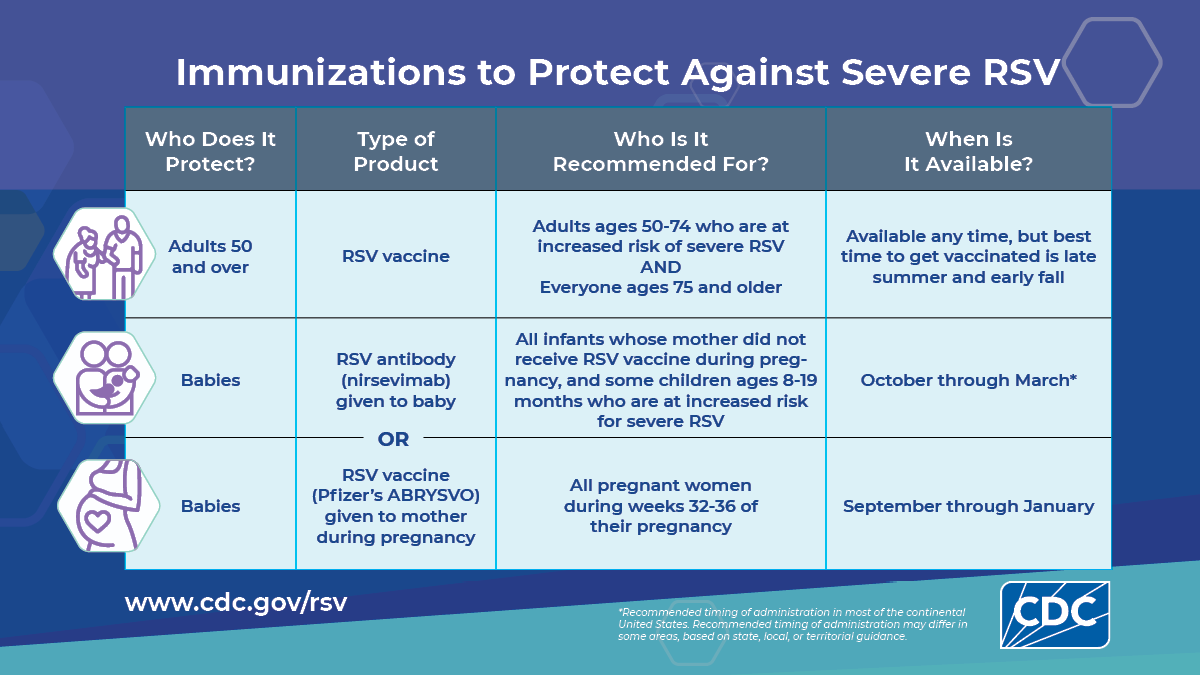
Products to protect against RSV became available for the first time last season. They're designed for older adults and babies, because they are the two groups most likely to get severely ill from RSV.
For older adults: Adults age 50-74 with health conditions, and adults 75+, should get vaccinated. This is not an annual vaccine; one dose is enough for now. CDC is studying how long protection lasts, so that may change. Current guidance is that protection lasts “at least two years.”
For babies: There are two strategies for protecting infants:
An antibody given to infants younger than 8 months during the winter RSV season. Protection lasts “at least 5 months.”
A vaccine given to pregnant women who are 32-36 weeks pregnant and plan to deliver between September and January. Protection will be passed on to the baby, and is expected to last 6 months.
"I hope this breakdown helps you navigate what's available this fall. The vaccine recommendations are confusing this year, particularly around Covid-19 eligibility, which will likely be limited compared to years past. I'll keep an eye on any major updates to guidance and share them if anything significant changes."

















.jpg)
No comments:
Post a Comment